Combines the speed and convenience you want with the strength and flexibility you need.
LiquiBand® Flex

Key Features
LiquiBand® Flex combines the speed and convenience you want with the flexibility and strength you need.
Key Benefits
Safe Applicator
- LiquiBand® Flex has a safe applicator with wings to aid further safety and control
Octyl-blend
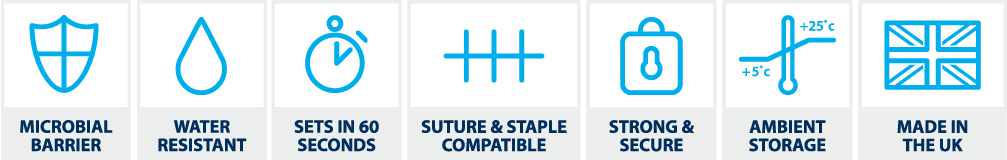
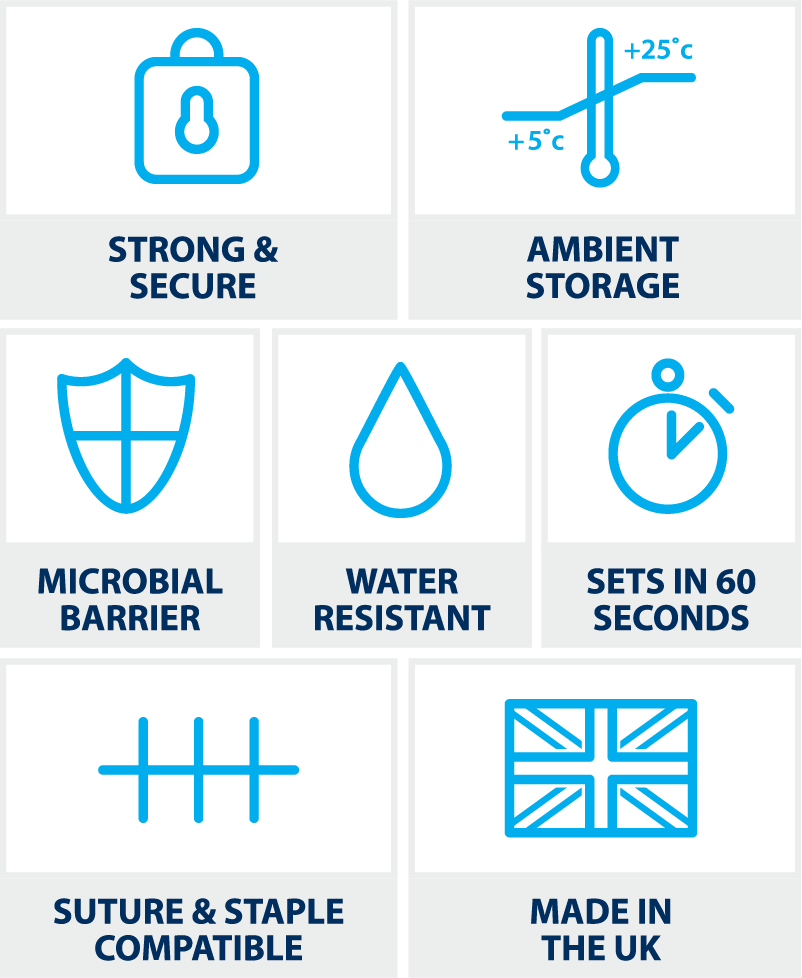
- LiquiBand® Flex contains an octyl-blend polymerising into a flexible fillm dressing providing strong and secure wound closure
Variety of surgical procedures
- LiquiBand® Flex is suitable for the topical closure of skin wounds which are a result of a variety of surgical procedures including: General & GI surgery (Laparascopy & Laparotomy), Plastic surgery, OBS & gynae (Episiotomy, C-Section), Neurosurgery (Craniotomy, shunts), Orthopedic surgery2
Fast set time
- LiquiBand® Flex sets on wounds in less than 60 seconds
Design Features
Design Features
1 Violet tint
- Makes it easy to see during application
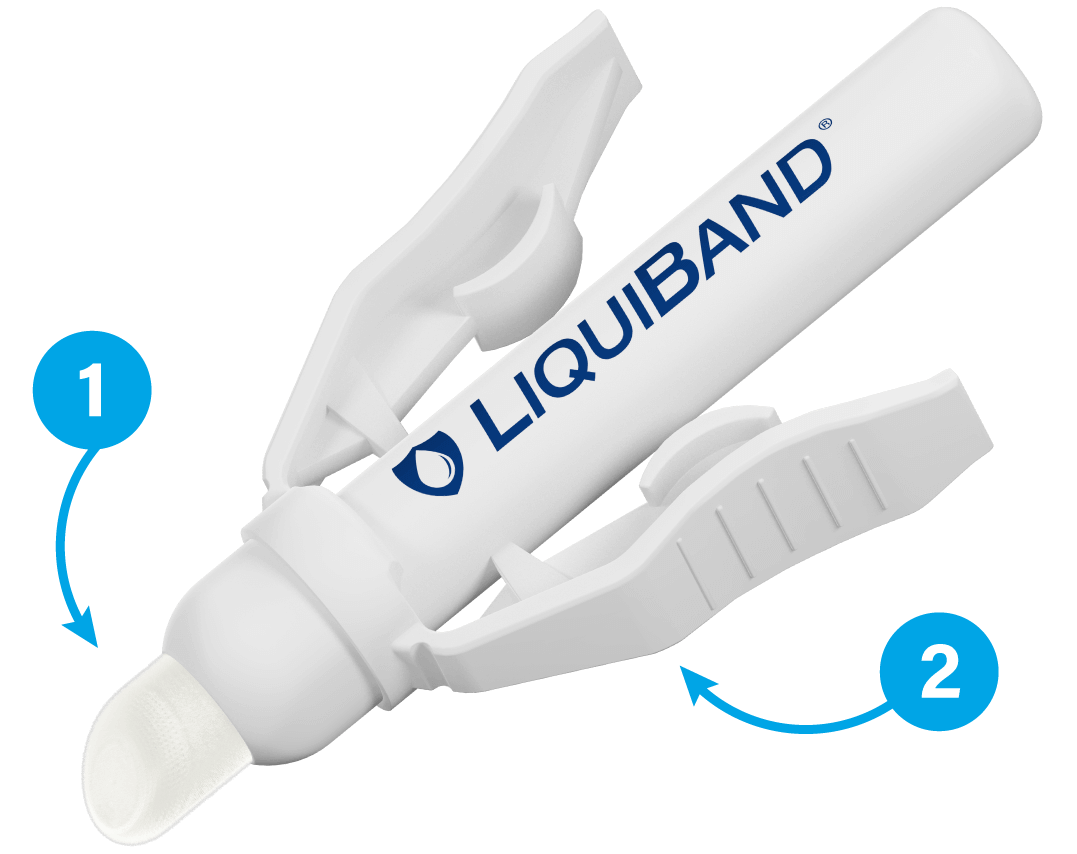
2 Easy to activate
- Unique, click-and-use winged applicator, removing the risk of glass shard injury
Only one application needed
- Ensuring adhesive stays intact throughout the wound healing process
- Naturally sloughs off within 5-10 days
Improved patient care
- Low exothermic reaction1
- Patient can shower immediately

Performance
Performance
High Tensile Strength
Superior tensile strength proven in a controlled bench study measuring the tensile strength of LiquiBand® Flex versus other topical skin adhesives
Newtons (N)
Microbial Barrier3
- Provides a strong, secure, and effective microbial barrier1
- Protection proven against Gram-positive, Gram-negative, and fungi microbes – locks out S. aureus, P. aeruginosa, E. coli, Candida albicans and MRSA3
Sizes & Codes
LiquiBand® Flex
| Product | Product Code | NHS SC Code | Volume per Each | Each per Box |
|---|---|---|---|---|
| LiquiBand® Flex | LBF 006 | FVF015 | 0.8g | 6 |